MEDICAL DEVICE-RELATED PRESSURE ULCERS/INJURIES
It is known that a significant proportion of pressure ulcers/injuries in critically ill or immobile patients are related to the use of medical devices (Black et al., 2010; EPUAP, 2019). A medical device–related pressure ulcer (MDRPU or DRPU) has been defined by Gefen et al., 2022 as:
‘A device-related pressure ulcer (DRPU) involves interaction with a device or object that is in direct contact with skin or is transdermally implanted under the skin, causing focal and localised forces that deform the superficial and deep underlying tissues. A DRPU, which is caused by a device or object, is distinct from a PU, which is caused primarily by body-weight forces. The localised nature of the device’s interaction with the patient’s tissue results in the appearance of skin and deeper tissue damage that mimics that of the device in shape and distribution.’
Adapted from AboutKidsHealth 2022
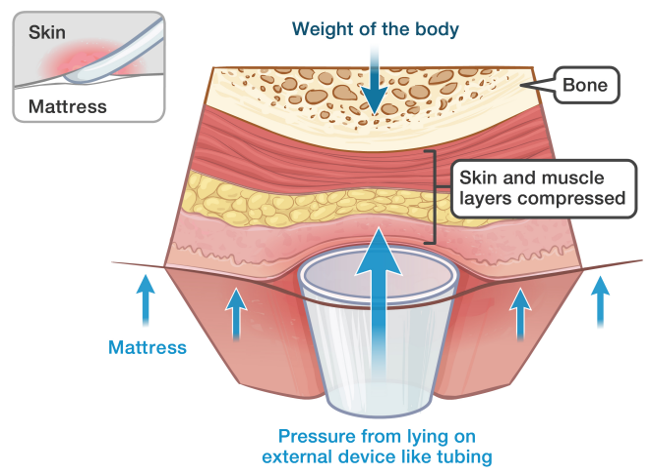
Prolonged exposure to mechanical loads can lead to device-related pressure injuries. Many medical devices that attach to the skin are based on generic designs that consist of stiff polymer materials, which are then secured in situ with tape or some form of strapping. The disparity between the mechanical properties of a stiffer device, soft skin and underlying tissues leads to focal deformations and mechanical stress near the contact sites with the device. Additionally, a medical device can result in an altered microclimate at the skin-device interface. (EUPAP, 2019; WUWHS, 2016)
The use of medical devices is essential across many healthcare settings, covering a wide range of products from simple bandages to the most sophisticated life-supporting products. The medical devices sector plays a crucial role in the diagnosis, prevention, monitoring, and treatment of diseases and the improvement of the quality of life of people suffering from disabilities (EWMA, 2020). However, patients with medical devices are approximately 2.4 times more likely to develop pressure ulcers/injuries of any kind (Black et al., 2010; EUPAP, 2019).
Many device-related pressure ulcers/injuries occur on the head or neck and are less frequently associated with a bony prominence, unlike non-device-related pressure ulcers, which occur more frequently below the waist (Apold & Rydrych, 2012).
| Location | Device | Non-Device |
|---|---|---|
| Head/face/neck | 70.3% | 7.8% |
| Other/multiple | 21.9% | 5.8% |
| Heel/ankle/foot | 20.3% | 16.9% |
| Coccyx/buttocks | 7.7% | 67.5% |
| Sacrum | 1.6% | 16.9% |
(Apold & Rydrych, 2012)
Devices commonly associated with medical device-related pressure ulcers/injuries include:
- Respiratory devices e.g. tracheostomy faceplates and securement devices masks used to deliver non-invasive positive pressure ventilation (NIV) or endotracheal (ET) and nasotracheal tubes
- Oximeter probes
- Oxygen tubing/nasal cannulas
- Orthopaedic devices, for example, cervical collars, halo devices, external fixators, braces, plaster casts
- Urinary/faecal collection devices e.g. indwelling urinary catheters, faecal continence systems
- Repositioning devices e.g. heel lifts, transfer board
- Device securements
- Nasogastric and feeding tubes
- Extra-corporeal membrane oxygenation (ECMO) cannulas
- Surgical drains
- Chest tubes
- Central venous and dialysis catheters
- Intravenous catheters and components
- Arterial lines
- Intra-aortic balloon pumps
- Retention sutures
- Blood pressure cuffs
- Intermittent pneumatic compression device sleeves
- Compression stockings and bandaging systems
- Restraints
- Devices and objects without a medical function e.g. mobile phones, objects left in the bed/chair
EUPAP, 2019; Gefen et al., 2022
On some occasions pressure damage is unavoidable as a consequence of saving the patient’s life (BHTVNF, 2010; NHS Midlands and East, 2012).
Patients at Risk of Pressure Damage
- Patients with impaired sensory perception
- Patients with communication difficulties
- Patients with reduced vascular status
- Patients with compromised skin integrity
- Patients with a medical device in situ
- Patients with oedema
(Wounds UK, 2014)
Hospitalised neonates are at risk for pressure ulcers/injuries due to immature skin, compromised perfusion, decreased mobility, altered neurological responsiveness, fluid retention, moisture, and medical devices.