Biofilms
Biofilms are complex microbial communities containing fungi and bacteria. The microorganisms synthesise and secrete a protective matrix that attaches the biofilm firmly to a living or non-living surface (Stoodley et al., 2002; IWII, 2022).
At a basic level, a biofilm can be described as bacteria embedded in a thick, slimy barrier of sugars and proteins. The biofilm barrier protects the microorganisms from external threats.
Biofilms have been long known to form on surfaces of medical devices, such as urinary catheters, implants, contact lenses, intrauterine devices and sutures (Costerton et al., 1999; Donlan & Costerton, 2002; IWII, 2022). Biofilms are also found in wounds and are suspected to delay healing. It is likely that all chronic wounds have biofilm communities on at least part of the wound bed (Phillips et al., 2010).
How do biofilms form?
- Reversible surface attachment - microorganism tend to attach to surfaces and eventually form biofilms. The initial attachment is reversible.
- Permanent surface attachment – as the bacteria multiply, they become firmly attached and differentiate, changing gene expression patterns.
- Slimy protective matrix/biofilms – once firmly attached, the bacteria begin to secrete a surrounding matrix known as extracellular polymeric substance (EPS). This is a protective matrix or ‘slime’. Small bacterial colonies then form initial biofilms.
(Phillips et al., 2010)
The composition of extracellular polymeric substances (EPS) varies according to the microorganisms present but generally consist of polysaccharides, proteins, glycolipids and bacterial DNA. Fully mature biofilms continuously shed planktonic bacteria, micro-colonies and fragments of biofilms, which can disperse and attach to other parts of the wound bed or to other wounds, forming new biofilm colonies (Costerton et al., 1999; Donlan & Costerton, 2002; Edward-Jones, 2016).
How quickly do biofilms form?
Studies have shown that planktonic bacteria such as Staphylococci, Streptococci, Pseudomonas and Escherichia coli, typically:
- Attach within minutes
- Form strongly attached micro colonies within 2-4 hours
- Develop EPS and become tolerant to biocides such as antibiotics, antiseptics within 6-12 hours
- Evolve into fully mature biofilms colonies and are resistant to biocides
- Rapidly recover from mechanical disruption and reform mature biofilms within 24 hours
(Phillips et al., 2010)
Chronic wounds often lack clinical signs of infection and have low bacterial burdens as measured by standard clinical microbiology laboratories. Standard clinical microbiology tests are optimised to culture planktonic bacteria, and do not adequately measure biofilms bacteria, which require special cultivation techniques.
Until standardised methods of recovering and detecting biofilm are developed, routine testing is currently unavailable (Edward-Jones et al., 2016).
To date, techniques used to detect the presence of a biofilms are:
- Scanning electron microscopy (SEM)
- Epiflourescent microsopy (EpiFM)
- Confocal laser scanning microscopy (CLSM)
- Detection quorum sensing molecule (QS)
- Molecular characterization of species present
At present these are only available in specialised research laboratories (Edward-Jones et al., 2016).
How do biofilms delay healing?
The exact mechanism by which biofilms impair healing of wounds is ambiguous (Bjarnsholt et al., 2017; IWII, 2022). Evidence suggests that biofilm keeps the wound in an inflammatory response, preventing normal wound healing. The pathways behind this are unclear as several systemic and local factors contribute to a chronic wound. In an acute wound a biofilm development leads to chronic inflammation.
At a local level, biofilm inhibits healing due to its relationship with the phenotypic abnormalities of epidermis and dermis derived cells in chronic wounds, as well as the physiology in an attempt to rid the wound of the biofilms. This response results in abundant neutrophils and macrophages surrounding biofilms. The inflammatory cells secrete high levels of reactive oxygen species (ROS), proteases (matrix metalloproteinases MMP’s) and elastase. The protease can assist in breaking down the attachments between the biofilms and the tissue, dislodging the biofilms from the wound. However, the ROS and proteases become chronically elevated and can accidentally (‘off target’) damage and destroy proteins that are essential for healing. This results in a chronic non-healing wound. (Philips et al., 2010; Bjarnsholt, 2017)
Criteria indicative of a potential biofilm:
- Failure of appropriate antibiotic treatment
- Recalcitrance to appropriate antimicrobial treatment
- Recurrence of delayed healing upon cessation of antibiotic treatment
- Delayed healing despite optimal wound management and health support
- Increased exudate/moisture
- Low-level chronic inflammation
- Low-level erythema
- Poor granulation/friable hypergranulation
- Secondary signs of infection
(IWII, 2022)
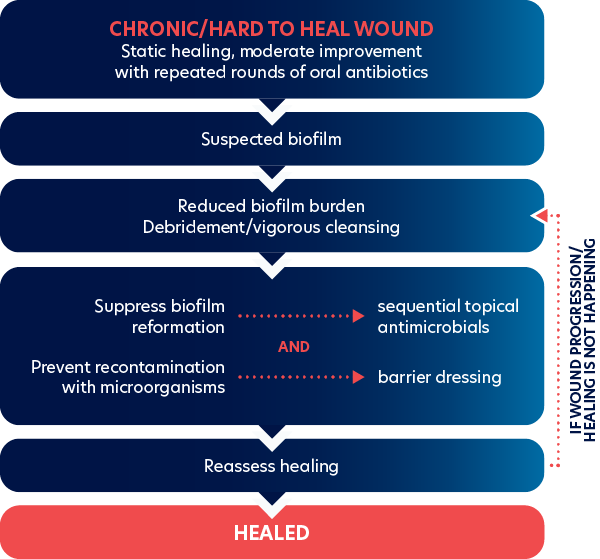
How can biofilm be reduced?
Evidence suggests that physical removal, debridement or physical cleansing are the best methods for reducing biofilm burden (Wolcott et al., 2009; IWII, 2022).
Biofilm removal is of clinical importance, requiring a multi-faceted approach, including physical removal through targeted wound hygiene, to eliminate. A debridement approach, together with therapeutic cleansing using topical surfactant and antiseptic solutions, with subsequent use of antimicrobial wound dressings, is recommended. A holistic management of the factors that can influence wound infection is advocated.
The goals of therapeutic cleaning and debridement in biofilm-based wound care are to:
- Physically remove the most tolerant microorganisms from the wound bed
- Create an environment that prevents or delays biofilm reformation
(WUWHS, 2016; Bjarnsholt, 2017; IWII, 2022)