Topic One: Larval Therapy
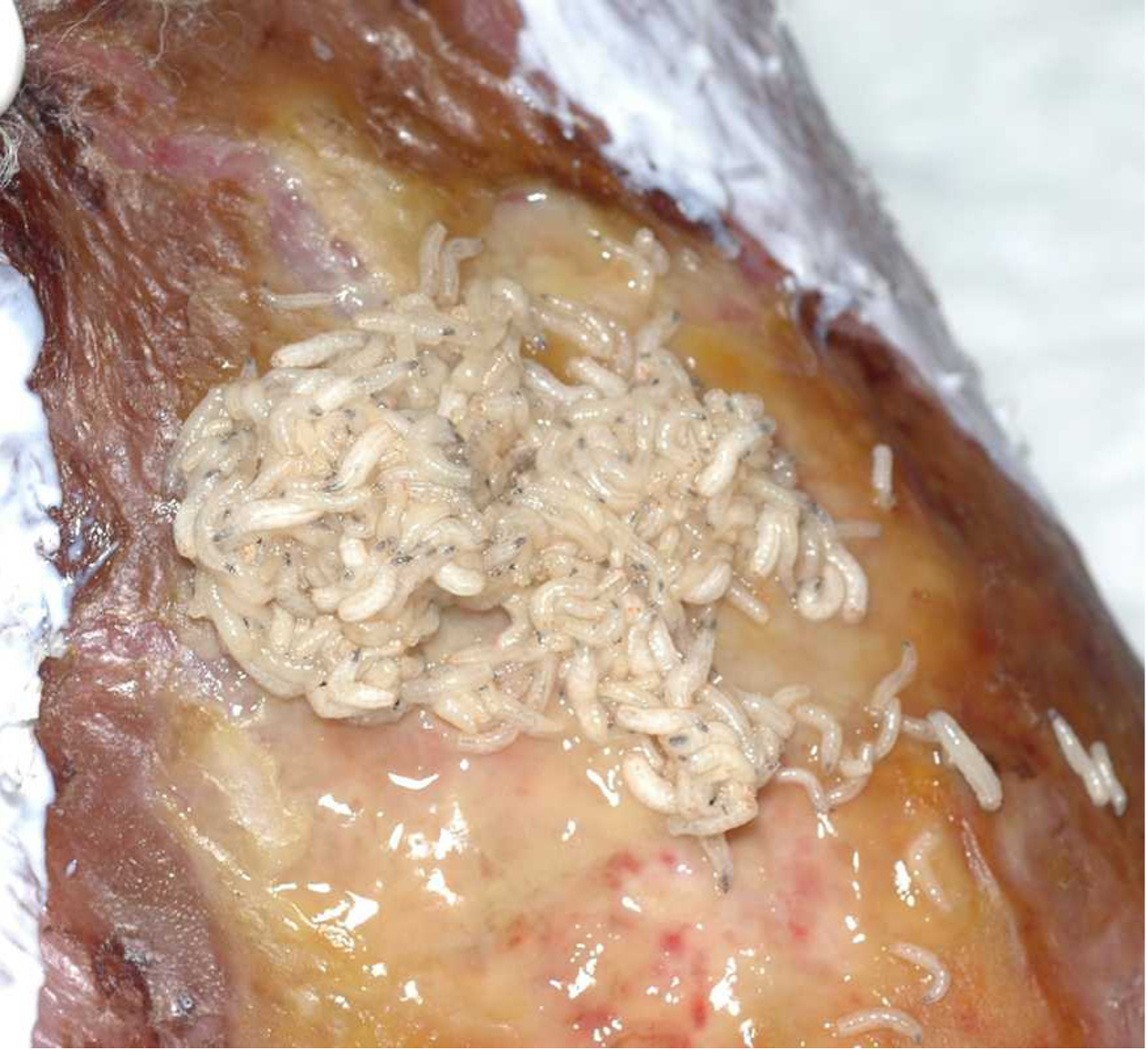
Maggot therapy, which is also referred to as biosurgery or larval therapy, has been used in wound healing for centuries. Baron Dominic Larrey, Napoleon’s Surgeon-in-chief, reported that when maggots developed in battle injuries they ‘prevented the development of infection and accelerated healing’ (Larrey, 1832).
The use of larvae (maggots) in wound healing was also documented during the American Civil War and the First World War. It became very popular during the 1930s, and numerous papers were published. Livingston attempted to combine maggot therapy with a polyvalent vaccine in an attempt to increase the healing success rate. However, this treatment caused systemic infections, and the use of maggots declined. It was at this time that antibiotics were also being introduced, which also caused a decline in the use of maggot therapy.
In the 1980s, the use of maggots was revived when Dr. Sherman used maggot therapy for treating pressure ulcers and other chronic wounds (Sherman & Pechter, 1988).
Larvae that are used in wound care are bred in a sterile environment and are free from bacterial contamination. The maggots are usually the larvae of the green bottle fly, Lucilia sericata.
The green bottle larvae live for approximately 21 days before they transform into a fly. Medical-grade maggots are 24 hours old when they are dispatched by the manufacturer to the requesting health care professional or patient. They should then be 48 hours old when applied to a wound. The larvae are usually removed within 3–4 days of application. It is not possible for the maggots to turn into flies while in the wound, and it is important to note that not all species of fly are suitable to be used for medicinal purposes. The larvae will not reproduce or multiply within the wound, as only adult flies lay eggs (Pagnamenta, 2013).
Mode of action
Larvae do not have any teeth and, as such, do not bite. The larvae move over the surface of the wound and secrete a mixture of proteolytic enzymes that break down the devitalised tissue in the wound. In effect, the larvae “drink” the liquefied tissue and bacteria (Vuolo, 2009; Pagnamenta, 2013).
As the larvae do not have teeth, it will take a longer period of time to break down any hard, dry necrotic tissue. Consequently, if larvae are placed in this type of wound, there is a high possibility that the larvae can die of starvation (Pagnamenta, 2013). Therefore, maggots are best used in sloughy, wet wounds.
The sterile maggots now used in wound healing will not attack or burrow into healthy human tissue (Vuolo, 2009).
Various studies have shown that larvae can kill or prevent the growth of microorganisms in wounds via two main mechanisms. Firstly, in 1935 Robinson et al., found that, during feeding, larvae ingest bacteria which are subsequently killed in the gut (Robinson, 1935). Secondly, the secretions that larvae produce as they move over the surface of the wound increase the pH of the wound to 8-8.5 due to the presence of ammonia. This increase in pH has an inhibitory effect on the growth of some bacteria (Messer & McClellan, 1935; Pavillard & Wright, 1957).
Larvae therapy is considered to be instrumental in the breaking down of necrotic tissue in chronic wounds and encourages healthy, granulating tissue in wound beds (Jones et al., 2011). As the larvae eat dead tissue, which can often be full of bacteria and also be malodorous, larvae are said to deodorise the wounds (Challinor, 2012). The digestive enzyme of the larvae means that it is safe to use around viable structures, such as tendon and bone. Furthermore, the enzyme is said to change the pH of the wound to a level where healing is stimulated and subsequently stimulates the growth of new blood vessels (Pagnamenta, 2013).
Indications for use
Larvae therapy is used to debride soft necrotic and sloughy tissue from a wound, including infected wounds (including those infected with MRSA) (Vuolo, 2009).
Larvae therapy is not commonly used as a first-line treatment. However, it can and often is considered for first-line use by an experienced specialist clinician, such as a Tissue Viability Nurse.
Larvae therapy may be used in the following wounds (if there is devitalised tissue present that has not been debrided through other techniques):
- Pressure ulcers
- Leg ulcers
- Diabetic foot ulcers
- Traumatic wounds
- Amputation sites
- Dehisced surgical wounds
- Infected wounds
(All Wales Tissue Viability Forum, 2013)
- Osteomyelitis
- Burns
- Abscesses
- Sub-acute mastoiditis
- Infected surgical wounds
- Necrotising fasciitis
- Malignant wounds
(Vuolo, 2009)
Please note: Maggots should only be considered for use by an experienced clinician.
*For dry hard eschar/necrotic tissue rehydration is required in the first instance. Refer to specialist and local policy
Wounds not suitable for larvae therapy include:
-
Those wounds where the blood supply is insufficient to permit healing, i.e. peripheral vascular disease
- Where the wound bed is covered by hard eschar (rehydration is required in the first instance)
- Wounds that connect with the body’s cavity or internal organs
- Fistulae that have not been probed or investigated
(All Wales Tissue Viability Forum, 2013)
It is fundamental that the health care professional has a comprehensive understanding of the procedure before commencing larval therapy. This would include:
- Suitable wound types
- Ethical considerations for the patient undergoing the therapy
- Contraindications and associated risks of treatment
- The potential for interaction with other medical products
The techniques required to administer both types of larval therapy are outlined by the manufacturer, with full instructions being included in each delivery of larvae. Health care professionals should be able to undertake a full wound assessment and create an effective plan of care. Involving the patient in the plan of care will ensure that all those involved have the same expectations of treatment (Griffin, 2014).
The use of maggot therapy should always be guided by an experienced clinician.
ADVERSE REACTIONS TO LARVAL THERAPY
Skin Irritation
As mentioned above, larvae secrete a mixture of proteolytic enzymes in order to break down devitalised tissue ready for ingestion. If these secretions come into contact with the skin that surrounds the wound, this can cause skin irritation. In order to prevent this, a larvae kit usually has a zinc-based barrier cream (Sudocream in the UK, for bagged larvae) or hydrocolloid (for loose larvae) to protect the periwound skin. These should be applied to the skin surrounding the wound prior to the application of larvae (Vuolo, 2009; All Wales Tissue Viability Forum, 2013; Griffin, 2014).
Pain and Discomfort
Some patients, particularly when maggots are used in the treatment of ischaemic limbs, may experience pain which can range from a ‘pricking’ sensation to very severe pain. The pain may result from the increase in wound pH and is normally ceased immediately when the maggots are removed (Courtenay & Church, 2000).
There is a perception of larval therapy, and a question often asked is if the larvae will be felt in the wound. This is doubtful. However, some individuals have described a tingling or stinging sensation. There is a possibility that those people with painful wounds may experience an increase in pain with larval therapy (Pagnamenta, 2013).
Some patients may develop pyrexia whilst undergoing maggot therapy (Courtenay & Church, 2000).
Less than 1% of wounds being treated with larvae will bleed. Sometimes, this bleeding can be heavy, possibly due to the erosion of a vessel wall (Courtenay & Church, 2000).
FACTORS INFLUENCING THE SURVIVAL OF LARVAE WITHIN A WOUND
There are factors that can affect or have been suggested might affect the survival rate of maggots within a wound.
Antibiotics
Studies have shown that if a patient is receiving normal therapeutic doses of antibiotics, this should not affect the growth and development of maggots (Sherman, Wyle & Thrupp, 1995).
Hydrogels
Often, wounds will have been treated with a hydrogel in an attempt to debride the wound prior to the decision to use maggot therapy. Some hydrogels contain propylene glycol, which kills maggots (Vuolo, 2009). Therefore, it is necessary to either thoroughly remove all traces of hydrogels containing propylene glycol prior to the application of maggots or to select a hydrogel that does not contain propylene glycol (Thomas & Andrews, 1999; Pagnamenta, 2013).
Excess Exudate
The survival and growth rate of maggots is reduced in the presence of excess liquid, possibly because their digestive enzymes become diluted and are less effective (Thornton, Berry & Ralston, 2002). This may explain why maggot therapy is sometimes not very effective in heavily exuding wounds.
X-Rays
X-rays do not have an adverse effect on the growth and development of maggots. Therefore, they do not need to be removed from a wound prior to X-ray investigations (Thomas, Jones, Shutler & Andrews, 1996).
It is essential to recognise that larvae are living creatures and, as such, care should be taken not to squash them. If they are applied to the heel or foot, there should be no weight bearing on that leg. For the same reason, it is not ideal to use maggots on sacral wounds unless the patient is positioned on their side for the duration of therapy. (All Wales Tissue Viability Forum, 2013)
It will be possible for the recipient of larvae therapy to carry out most normal activities. It is advisable to refrain from bathing as the wound should not be immersed in water. The wound must not be placed too close to a heat source, e.g. a living room fire or radiator, as the larvae may dry out and die (Pagnamenta, 2013).
Ethics
There are no major ethical issues relating to the use of maggots, and no special permission is required. It is important that the patient fully understands the treatment and is able to give consent.
To conclude, maggot or larval therapy is a safe and highly effective method for debriding many different types of wounds but should only be undertaken following consultation with the Tissue Viability Service or other specialist health care professionals.